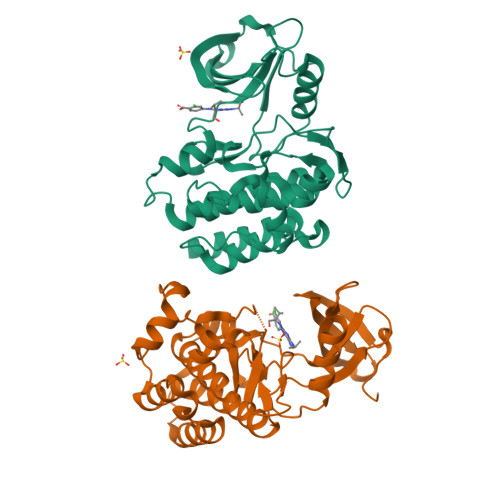
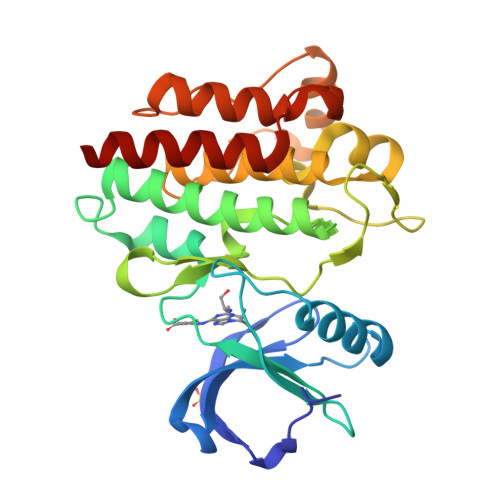
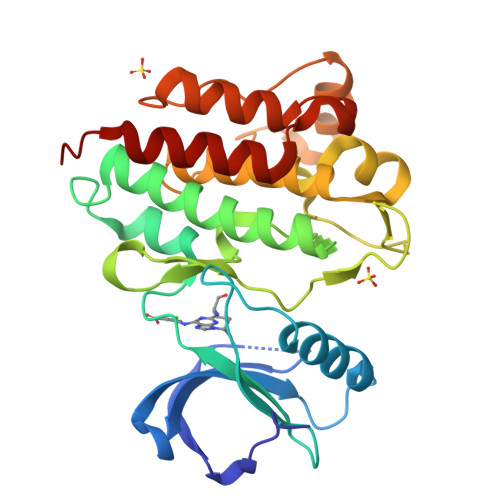
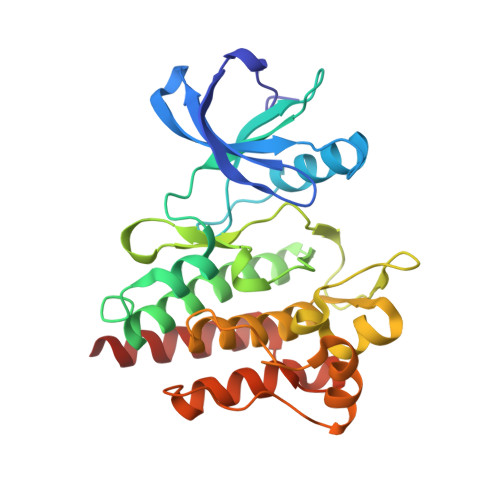
Predicting the Conformational Variability of Abl Tyrosine Kinase using Molecular Dynamics Simulations and Markov State Models.
Meng, Y., Gao, C., Clawson, D.K., Atwell, S., Russell, M., Vieth, M., Roux, B.(2018) J Chem Theory Comput 14: 2721-2732
- PubMed: 29474075
- DOI: https://doi.org/10.1021/acs.jctc.7b01170
- Primary Citation of Related Structures:
6BL8 - PubMed Abstract:
Understanding protein conformational variability remains a challenge in drug discovery. The issue arises in protein kinases, whose multiple conformational states can affect the binding of small-molecule inhibitors. To overcome this challenge, we propose a comprehensive computational framework based on Markov state models (MSMs). Our framework integrates the information from explicit-solvent molecular dynamics simulations to accurately rank-order the accessible conformational variants of a target protein. We tested the methodology using Abl kinase with a reference and blind-test set. Only half of the Abl conformational variants discovered by our approach are present in the disclosed X-ray structures. The approach successfully identified a protein conformational state not previously observed in public structures but evident in a retrospective analysis of Lilly in-house structures: the X-ray structure of Abl with WHI-P154. Using a MSM-derived model, the free energy landscape and kinetic profile of Abl was analyzed in detail highlighting opportunities for targeting the unique metastable states.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology , University of Chicago , Chicago , Illinois 60637 , United States.